Chain-Growth versus Step-growth Polymerization
| Step-Growth | Chain-Growth |
| All molecules present (monomer, oligomer, polymer) can react with any other molecule. | During propagation, only monomers react to the active site at the end of the growing chain. |
| Monomers exist throughout the reaction, but large quantities of monomers are consumed early in the reaction. | Monomers exist throughout the reaction; its concentration decreases steadily with time. |
| There is no termination step and the end groups of the oligomers and polymers are reactive throughout the polymerization process. | There are two distinctive mechanisms during polymerization; these are initiation and propagation. In most cases there is also a termination step. |
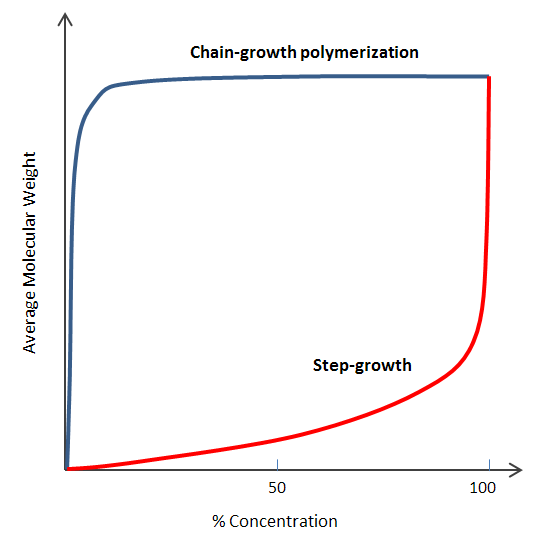
| The reaction
proceeds rapidly at the beginning but the molecular weight increases only slowly and high MW's are only attained at
the end of the process by long oligomers reacting with each-other. |
The reaction speed depends on the concentration of initiator (and co-initiator) and high-molecular weight polymers form throughout the duration of the reaction. |
| Long reaction times are needed for the synthesis of long (high molecular weight) polymers. | Long reaction times have high degrees of conversion but do not affect (much) the (average) molecular weight. |
| Molecular species of any length (oligomers) exist throughout the reaction, with the length distribution broadening and shifting to higher MW with increasing reaction time. | The mixture contains primarily monomers and polymers, and only small amounts of growing polymer chains; |
Step-growth versus Chain-growth Polymerization